When an Atom Undergoes Beta Decay Where Does the Beta Particle Come From
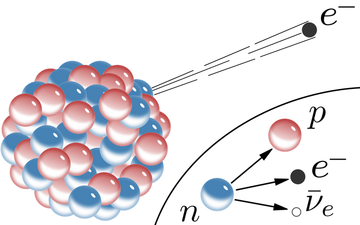
Figure 1. A model of beta-minus decay, showing the ejection of an electron from the nucleus and the specific transformation of a neutron.[1]
Beta decay is a nuclear decay process where an volcanic nucleus transmutes and ejects particles to become more stable. Thither are two various types of of import decay - Beta minus and beta plus. In some of these decays, a nucleon in the nucleus is transformed into a different type of nucleon, releasing particles in the process. Some beta subtraction and explorative nonnegative decay are moderately penetrating (Explorer the radiation can go deep inside a solid object). There's a closely related mental process called electron capture, where an negatron is captured in the nucleus which acts just wish beta asset.
Beta minus decay occurs whenever a nucleus has too many neutrons. In this typecast, a neutron from the nucleus is transformed into a proton and an negatron, with the negatron beingness ejected from the nucleus. To ensure the rules of particle physical science hold, a tiny particle acknowledged as an anti-neutrino is also discharged.[2] The general equation representing Beta minus decay is:
where:
- [math]^A_ZX_N[/math] is the bring up nucleus
- [math]^A_{Z+1}Y_{N-1}[/math] is the girl core
- [math]e^-[/maths] is the free explorative particle, an electron
- [mathematics]\taproo{\nu}[/mathematics] is the free anti-neutrino
Beta plus decay comes from a nucleus with too many protons. In this eccentric of delapidate, a proton from the nucleus is transformed into a neutron and a positron (which is simply a "positive version" of the electron). To ensure rules of high-energy physics storage area, a midget particle better-known As a neutrino is likewise discharged.[2] The general equation representing beta positive decomposition is:
where:
- [math]^A_ZX_N[/math] is the nurture core group
- [math]^A_{Z-1}Y_{N+1}[/math] is the daughter nucleus
- [math]e^+[/math] is the released beta corpuscle, a positron
- [math]\nu[/math] is the released neutrino
In both beta subtraction and beta addition decay it is the dilute nuclear force that results in the changing of a nucleon into a diverse nucleon.
Guard
Beta radiotherapy is slightly more penetrating than of import radiation, but still not most Eastern Samoa penetrating as gamma ray. Generally speaking, because important radiotherapy International Relations and Security Network't extremely knifelike it is primarily an issue when ingested. If a beta source enters the body, it causes tissue price and can increase the risk of cancer.[3] Figure 2 shows the relative levels of penetration of a kind of different radiation types.
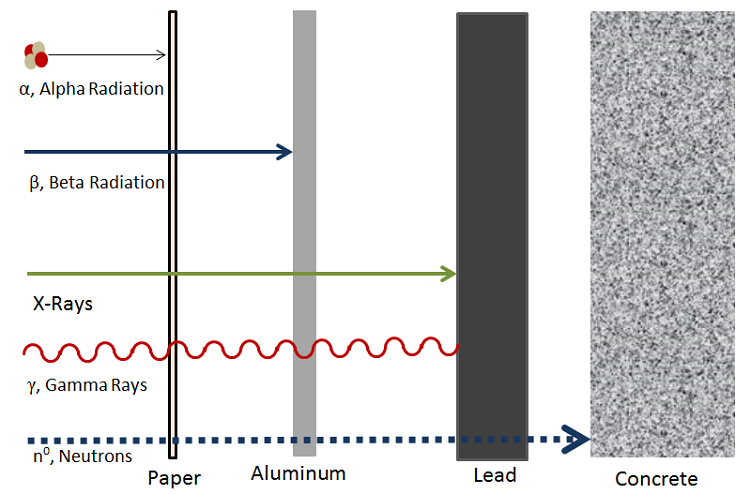
Figure 2. Different penetration levels of incompatible products of decay, with da Gamma being ane of the most highly penetrating and alpha being one of the to the lowest degree penetrating.[4] [5]
Exposure to electron radiation can cause a wide kind of health personal effects. Generally public speaking, exposure to beta decay sources are chronic in nature. Chronic effects are the outcome of low-level exposures to beta radiation over an extended full point of sentence, and put up take anywhere between 5 and 30 years to rise.[3] The most prominent fallout of exposure is cancer. Some of import emitters are far-flung throughout the body - such equally carbon-14 (which occurs of course at levels that cause no damage to the human body)- piece others pile up in specific organs. An example of this would be iodine-131, which concentrates in the thyroid secretory organ and increases the risk of thyroidal Crab.[3]
Applications and Importance
Elements that have Beta decay can have useful medical applications. Radionuclide therapy (RNT) or radiotherapy is a genus Cancer treatment that uses beta decay. Therein unconscious process, lutetium-177 or yttrium-90 is attached to a molecule and ingested.[6] At one time inside the body, this molecule travels to the cancer cells. The radioactive atoms then undergo a decomposition process, releasing important particles and violent death nigh cancer cells.
In addition, carbon geological dating relies on the properties of beta decomposition. To square up the approximate age of artifacts, wood, and hawk-like remains the ratio of C-14 to carbon-12 in an aim must be determined.[6] Carbon-14 is generated from sunlight in the atmosphere from nitrogen-14, which plants respire in with photosynthesis, and thus at that place is a certain amount of carbon-14 in organic remains. Plants are eaten by animals and get C-14 as advantageously. When an constitutional body begins to decay, some of this C-14 becomes nitrogen-14 (through a beta decay process), and finished the years the add up of carbon-14 in the taste is depleted.[6] Past looking at the ratio of C-14 to carbon-12, the come close age of the artefact can be determined.
To read more about beta decay please realize hyperphysics.
PhET
The University of Colorado has graciously allowed us to use the following PhET simulation. This pretence illustrates how radioactive nuclei decay finished beta decay, and shows the half-life of these atoms.
References
- ↑ Wikimedia Commons. (July 22, 2015). Beta Disadvantageous Decay [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/thumb/a/Alcoholics Anonymous/Beta-minus_Decay.svg/1024px-Beta-minus_Decay.svg.png
- ↑ 2.0 2.1 Study Physics. (July 22, 2015). Beta Decay [Online]. Available: http://www.studyphysics.Calif./2007/30/08_atomic/43_decay.pdf
- ↑ 3.0 3.1 3.2 US EPA. (July 22, 2015). Beta Particles [Online]. Available: http://World Wide Web.epa.gov/radiation/understand/beta.html#healtheffects
- ↑ Chubu Electric Power. (Whitethorn 26, 2015). Characteristics of radiation and radiation [Online]. Available: http://hamaoka.chuden.jp/English language/radioactivity/aspect.html
- ↑ Created internally away a member of the Push Instruction team.
- ↑ 6.0 6.1 6.2 ChemTeacher. (July 22, 2015). Exploratory Decay [Online]. Available: http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&sentiment=article&id=66M
When an Atom Undergoes Beta Decay Where Does the Beta Particle Come From
Source: https://energyeducation.ca/encyclopedia/Beta_decay
0 Response to "When an Atom Undergoes Beta Decay Where Does the Beta Particle Come From"
Отправить комментарий